Phases of Batch Vacuum Drying of Solids
Vacuum drying of solids provides several advantages including lowering of the vaporization temperature, protecting the product from excess heat, increased thermal efficiency and recovery of vapors. By drawing vacuum on the vessel, the temperature at which the water or solvent will evaporate will be reduced. This vaporization of the liquid will remove the heat from the solids and thus provide evaporative cooling, protecting heat sensitive products.
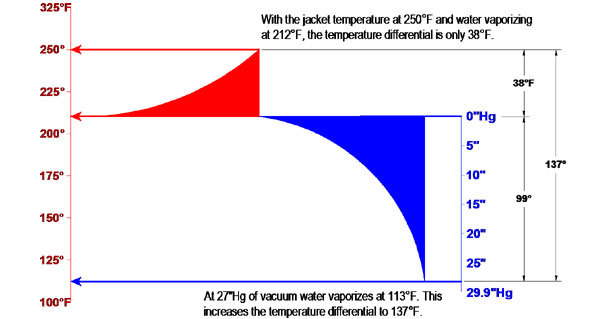
The lowered vaporization temperature will also improve the temperature differential between the product and the heated jacket. This increases the driving force and will shorten the drying time. For example, at atmospheric pressure water vaporizes at 212°F. At 27”Hg of vacuum, water will vaporize at 113°F. No matter what the jacket temperature may be, this lowering of the vaporization temperature will increase the temperature differential an additional 99°F. As long as there is unbound moisture to freely evaporate, the solids will not exceed 113°F. The graphic below shows the temperature difference a jacket at 250°F and water evaporating at 212°. That is just a differential of 38°F. By increasing the vacuum to 27”Hg, the water vaporizes at 113° and the temperature differential is now 137°F. That is 3.6 times more driving force for heat transfer and driving off the water.
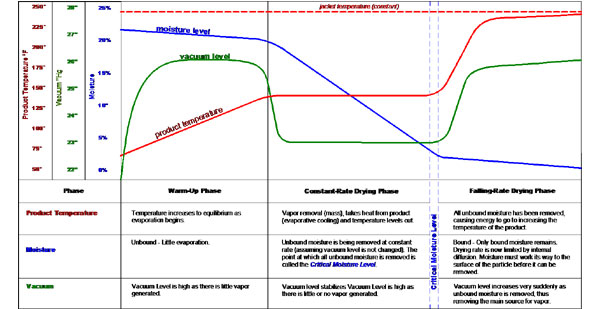
Batch vacuum drying of solids goes through three phases. During these three phases, the thermal, vapor and drying characteristics will change in predictable ways.
The first phase of vacuum drying is the warm-up phase where the initial heating of the vessel and the product to be dried including the solids and liquids occurs. During this warm-up phase the system temperature increases steadily and in a predictable fashion. The heat input is known as sensible heat because the heat input can be sensed and measured as an increase in temperature.
In the second phase of vacuum drying most of the heat energy goes to the vaporizing the liquid. The moisture coming off here is known as unbound moisture or free moisture. Because this moisture comes off as fast as the heat energy input, the heat is removed by the vaporization of the moisture and provides an evaporative cooling effect. This phase is known as the constant-rate drying phase because the unbound moisture will come off at a constant rate. As can be seen in the graph below, the system temperature and the vacuum level remain constant and all of the additional energy going to the system (aside from some heat loss to the surrounding air) will be going into the vaporization of the liquid. This heat energy is known as latent heat (latent is Latin for “hidden”), because no further temperature increase takes place. During this phase the condenser load will be at is highest and constant.
The third phase of vacuum drying is known as the falling-rate drying phase because the rate at which moisture is removed will continue to fall or decrease with time. The falling-rate phase occurs after the unbound moisture has been removed, leaving only the bound moisture in the product. This moisture is bound in the product and must work its way to the surface of the solids by capillary action or diffusion before it can evaporate and be removed. This moisture will eventually be removed, but with some added effort and time. In some processes such as polymer drying, the bound moisture may take many hours or even a day or two. The diffusion of moisture simply cannot be pushed past a certain point. On the other hand, solids such as minerals may have mainly surface moisture at little to no bound moisture. Once we are down to the bound moisture, there no free moisture to take the heat away from the product so the temperature of the product will start to rise and approach the temperature of the jacket. This temperature rise of the product may have to be compensated for by lowering the jacket temperature in the case of heat sensitive products. During the falling-rate phase, relatively little moisture will be removed so the load on he condenser will be minimized. Likewise, the thermal load will be fairly low as little moisture is vaporized per unit time.
The point in the process between where the unbound moisture has been removed and only bound moisture remains is known as the critical moisture level. The critical moisture level represents the divide between the constant-rate phase and falling-rate phase of the drying process. This is very valuable in laboratory and production operation and can be easily recognized by the pronounced increase in product temperature and increase in vacuum level (provide the vacuum level was not already at maximum due to design).
See our Vacuum Drying Options